Main Difference – 4f vs 5f Orbitals
Atoms are composed of a nucleus that is made out of protons and neutrons, which are surrounded by electrons. These electrons are in continuous movement around the nucleus. Therefore, we cannot give a specific location for an electron in an atom. Instead of locating the exact position of an electron, scientists have introduced the concept of “probability.” In other words, the most probable pathway that an electron is most likely to be moving is determined. This pathway is called an orbital. There are different subsets of orbitals such as s orbitals, p orbitals, d orbitals and f orbitals. The number of orbitals in each subset is determined by the magnetic quantum number. For f orbitals, there are 7 possible magnetic quantum numbers, so there are seven f orbitals. 4 f and 5 f orbitals are the first set and second set in f orbitals. The main difference between 4f and 5f orbitals is that 4f orbitals have a number of planes and conical nodes, but no radial nodes whereas 5f have a number of planes and conical nodes, and each orbital have one radial node.
Key Terms: Atom, Electron, Lobes, Magnetic Quantum Number, Nodes, Nucleus, Orbital, Probability, Subshell
4f orbitals are the seven f orbitals of the 4th electron shell (energy level). 4f orbitals are the first subset of f orbitals. This means 1st, 2nd and 3rd electron shells have no f orbitals. This is shown below in the table of s, p, d and f orbitals.
|
Electron Shell |
Orbitals |
|
1 |
s |
|
2 |
s, p |
|
3 |
s, p, d |
|
4 |
s, p, d, f |
|
5 |
s, p, d, f |
A set of 4f orbitals has four different shapes, each having a number of planar and conical nodes. But 4f orbitals possess no radial nodes. The seven 4f orbitals are named according to the plane of the orbital. Given below are the seven 4f orbitals.
Among these orbitals, both 4fxyz and 4fz(x2-y2) orbitals have eight lobes. They are related to each other by a 45o rotation around the z axis. This means, they are similar in other factors but are different in the plane.
Among the rest of the seven orbitals, 4fy(3×2-y2) and 4fx(x2-3y2) orbitals are related to each other by rotating 90o around the z axis. Each orbital has six lobes separated by three nodal planes having an angle of 60o among them. The 4fxz2 and 4fyz2 orbitals seem similar to 4fy(3×2-y2) and 4fx(x2-3y2) orbitals but are different because the three nodal planes of six planes are not separated by 60o angles. These 4fxz2 and 4fyz2 orbitals have two of the six lobes “bean-shaped” in z axis. On the other hand, 4fxz2 orbital is a similar orbital to that of 4fxz2 and 4fyz2 orbitals, but the “bean-shaped” lobes are in the x axis. In 4fyz2 orbital, the “bean-shaped” orbital is in the y axis.
5f orbitals are the seven f orbitals of the 5th electron shell (energy level). 5f orbitals are the second subset of f orbitals. These orbitals are named based on the planes of orbitals. The seven orbitals are as follows.
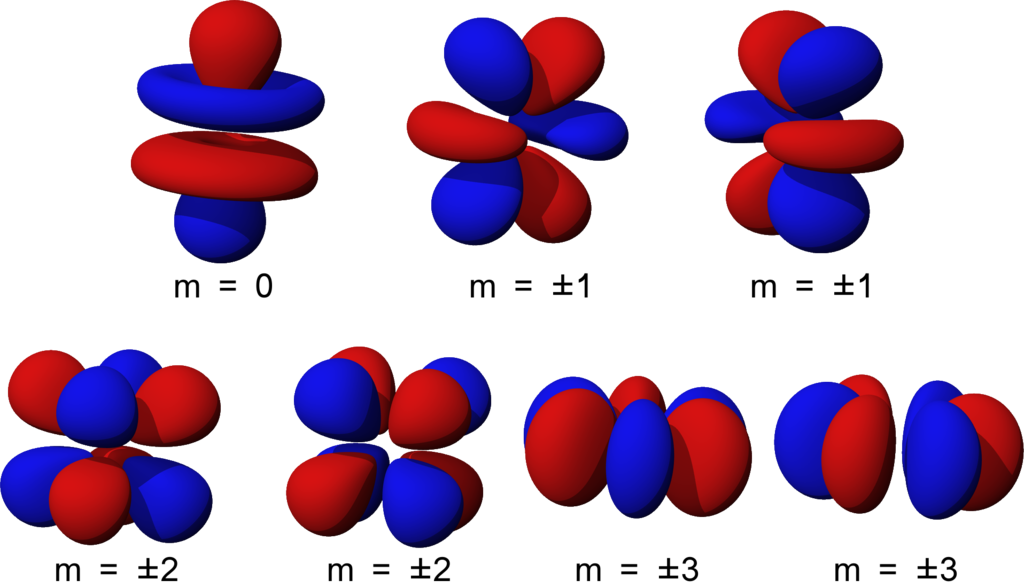
A set of 5f orbitals has four different shapes, each having a number of planar and conical nodes. Each 5f orbital also possesses one radial node as well.
Among these orbitals, both 5fxyz and 5fz(x2-y2) orbitals have eight lobes. They are related to each other by a 45o rotation around the z axis. This means, they are similar in other factors but are different in the plane to which they are directed.
Among the rest of the seven orbitals, 5fy(3×2-y2) and 5fx(x2-3y2) orbitals are related to each other by rotating 90o around the z axis. Each orbital has six lobes separated by three nodal planes having an angle of 60o among them. The 5fxz2 and 5fyz2 orbitals seem similar to that of 5fy(3×2-y2) and 5fx(x2-3y2) orbitals, but they are different because the three nodal planes of six planes are not separated by 60o angles. These 5fxz2 and 5fyz2 orbitals also have two of the six lobes “bean-shaped”. On the other hand, the 5fxz2 orbital is a similar orbital to that of 5fxz2 and 5fyz2 orbitals, but the “bean-shaped” lobes are on the x axis. In 5fyz2 orbital, the “bean-shaped” orbital is in the y axis.
with f subshell, l=3 so ml =-3,-2, -1 , 0 ,+1,+2,+3 so there are seven possible values of ml .Remember that 4 is the number of principal quantum number it has nothing to do with the number of the orbitals. So, there will seven 4f orbitals . Answer
Each of the orbitals is named for the expression based upon x, y, and z in the angular wave function, but some abbreviated names are useful for simplicity. These are:
The radial equations for all the 4f orbitals are the same. The real angular functions differ for each and these are listed above.
For s-orbitals the radial distribution function is given by 4Ïr2Ï2, but for non-spherical orbitals (where the orbital angular momentum quantum number l > 0) the expression is as above. See D.F. Shriver and P.W. Atkins, Inorganic Chemistry, 3rd edition, Oxford, 1999, page 15. The OrbitronTM, a gallery of orbitals on the WWW:
For any atom, there are seven 4f orbitals. The f-orbitals are unusual in that there are two sets of orbitals in common use. The cubic set is appropriate to use if the atom is in a cubic environment. The general set is used at other times. Three of the orbitals are common to both sets. These are are the 4fxyz, 4fz3, and 4fz(x2-y2) orbitals.
FAQ
How many 4f orbitals are there in an atom What is the maximum number of electrons possible in a set of 4f orbitals?
Do 4f orbitals exist?
How many orbitals are in 4d?