Step #3: Put two electrons between the atoms to represent a chemical bond
Now in the above sketch of HNO molecule, put the two electrons (i.e electron pair) between the hydrogen atom, nitrogen atom and oxygen atom to represent a chemical bond between them.

These pairs of electrons present between the Hydrogen (H), Nitrogen (N) and Oxygen (O) atoms form a chemical bond, which bonds these atoms with each other in a HNO molecule.
Step 2: Select the central atom
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
(Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.)
Now here the given molecule is HNO and it contains hydrogen atom (H), nitrogen atom (N) and oxygen atom (O).
So as per the rule we have to keep hydrogen outside.
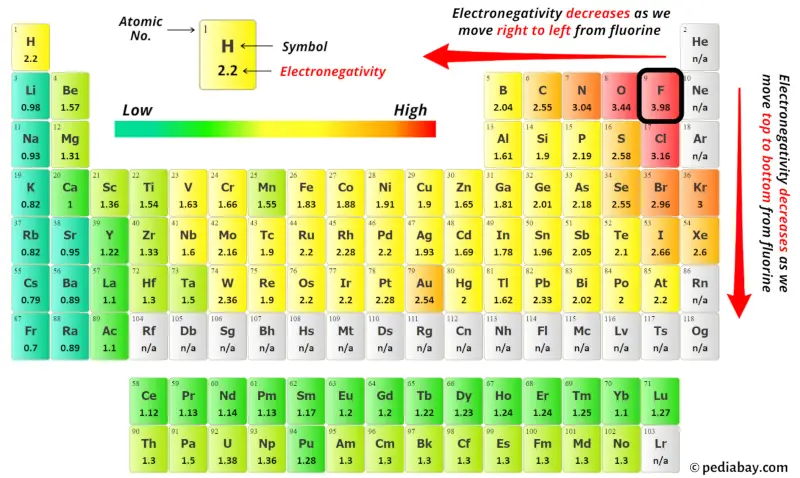
Now, you can see the electronegativity values of nitrogen atom (N) and oxygen atom (O) in the above periodic table.
If we compare the electronegativity values of nitrogen atom (N) and oxygen atom (O) then the nitrogen atom is less electronegative.
So here the nitrogen atom is the center atom and the oxygen atom is the outside atoms.
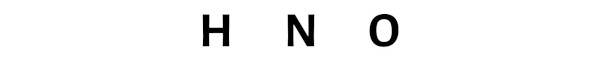
Step #4: Complete the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom
In the Lewis structure of HNO, the outer atoms are hydrogen atom and oxygen atom.
Hydrogen already has a duplet (see below ).
So now, you have to complete the octet on oxygen atom (because oxygen requires 8 electrons to have a complete outer shell).
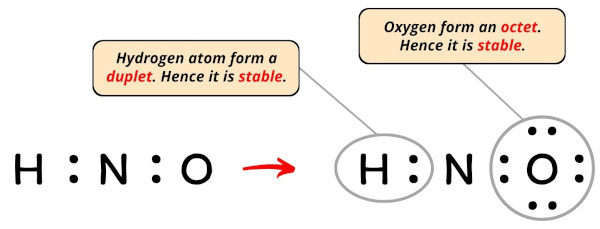
Now, you can see in the above that the oxygen atom forms an octet.
Also, only 10 valence electrons of HNO molecule are used in the above structure.
But there are total 12 valence electrons in HNO molecule (as calculated in step #1).
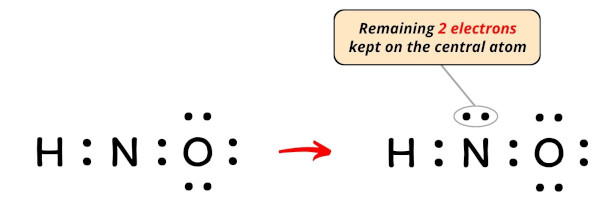
So the number of electrons left to be kept on the central atom = 12 – 10 = 2.
So let’s keep these two electrons (i.e 1 electron pair) on the central atom.
Now, let’s move to the next step.
FAQ
What is the lewis structure of HNO?
What is HNO shape?
How many lone pairs of electrons are on the central atom in HNO?
How many valence electrons does HNO have?