The Iodine pentafluoride chemical formula is IF5. Drawing IF5 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct IF5 Lewis Structure. The Iodine and fluorine elements come as the member of the halogen family group from the periodic table. The valence electrons in Iodine and fluorine are seven. The branch of halogen chemistry is used to make chemicals reagents for fluorination reactions.
Step #1: Draw the lewis structure
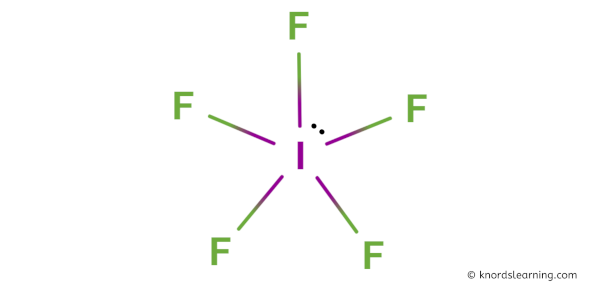
Here is a skeleton of IF5 lewis structure and it contains five I-F bonds.
(Note: If you want to know the steps of drawing the IF5 lewis dot structure, then visit this article: IF5 lewis structure).
So from the above diagram we have come to know that the IF5 molecule has five I-F bonds.
Now in the next step we have to check whether these I-F bonds are polar or nonpolar.
And we also have to check the molecular geometry of IF5.
What type of bond is IF5?covalent bond If5, also known as iodine pentafluoride ) is covalent bond list below antimony has a slight charge… The greatest electronegativity, each F has a slight negative charge a square base surrounding a central iodine.. Difference is very small or zero, the bond is covalent and nonpolar SbCl3 have about %…
Does IF5 have a dipole moment?Answer and Explanation: Iodine pentafluoride has a dipole moment, which is a measure of its polarity. The fluorine molecules are more electronegative than the iodine, which…
Is CCl4 a polar or nonpolar molecule?The molecule of CCl4 is nonpolar in nature because of its symmetrical tetrahedral structure. However the C-Cl bond is a polar covalent bond, but the four bonds cancel the polarity of each other and form a nonpolar CCl4 molecule.
Is BrCl3 polar or nonpolar?BrCl3: the molecule has a T-shaped molecular geometry which could make it polar, but the Br-Cl electronegativity difference is 0.2 which makes it a covalent bond. CS2: the molecule has a linear geometry and the electronegativity difference between C and S is 0, so this one is not polar either. Apr 18, 2013
Is carbon disulfide polar or nonpolar?Carbon disulfide is not a polar molecule. Electronegativity is the measure of how strongly an atom will attract electrons to itself.
Is ammonia polar or nonpolar?Ammonia is a polar molecule: The electrostatic potential clearly shows that the nitrogen is partially negative whereas the hydrogens are partially positive.
Is C2H4 polar or nonpolar molecule?Conclusion. Ethylene (C2H4) is a linear-shaped molecule with a double bond between both carbon atoms (C=C). The C-H bond is also nonpolar because of nearly the same electronegativity. As a result, the entire molecule is nonpolar.
Is CL more electronegative than C?Check out boron: it’s less electronegative than hydrogen [2.0 vs. 2.2]. … Carbon is More Electronegative Than You Think. Element Electronegativity (Pauling) Cl 3.2 [3.16] N 3.0 [3.04] Br 3.0 [2.96] I 2.7 [2.66] 9 more rows • Mar 7, 2010
Why is C CL more polar than CF?The C-F bond is more polar than C-Cl bond because F is more electronegative than Cl. … And also the polarity also depends on bond length and since due to the greater size of Cl atom the bond length is less than F and hence the low polarity.
Is CC more polar than CH?The C−H bond is indeed slightly more polar than the C−C bond, but this slightly increased polaity can in no solely way account for the approximately 20% increase in bond energy. In fact, Wikipedia writes: “Because of this small difference in electronegativities, the C−H bond is generally regarded as being non-polar.”
Is CCl4 a dipole?Carbon tetrachloride,CCl4, has a net dipole moment of zero. Even though each of the four C-Cl bonds is distinctly polar, the resultant moment of any three of them is equal in magnitude but opposite in direction to the moment due to the fourth one. So, the molecule’s net dipole moment is zero, and it is non-polar. Mar 7, 2020
FAQ
What best describes the polarity of IF5?
Is IF5 have a dipole moment?
What is the electronegativity of IF5?