Steps of drawing XeH4 lewis structure
In order to find the total valence electrons in XeH4 molecule, first of all you should know the valence electrons present in xenon atom as well as hydrogen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Here, I’ll tell you how you can easily find the valence electrons of xenon as well as hydrogen using a periodic table.
Total valence electrons in XeH4 molecule
→ Valence electrons given by xenon atom:
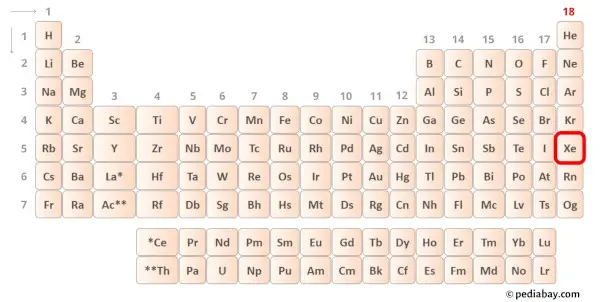
Xenon is a group 18 element on the periodic table. Hence the valence electrons present in xenon is 8.
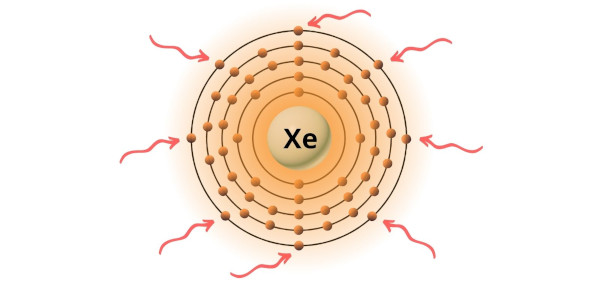
You can see the 8 valence electrons present in the xenon atom as shown in the above .
→ Valence electrons given by hydrogen atom:
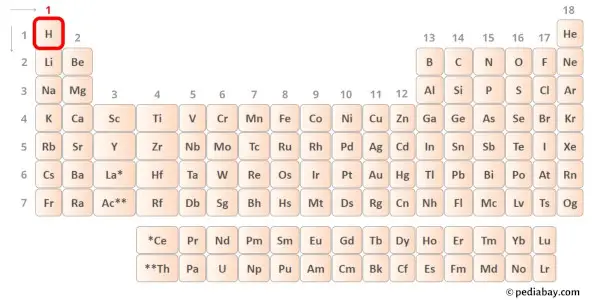
Hydrogen is group 1 element on the periodic table. Hence the valence electron present in hydrogen is 1.
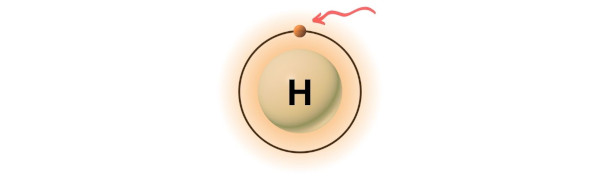
You can see that only 1 valence electron is present in the hydrogen atom as shown in the above .
Total valence electrons in XeH4 molecule = valence electrons given by 1 xenon atom + valence electrons given by 4 hydrogen atoms = 8 + 1(4) = 12.
Step #2: Select the center atom (H is always outside)
While selecting the center atom, always put the least electronegative atom at the center.
Here in the XeH4 molecule, if we compare the xenon atom (Xe) and hydrogen atom (H), then hydrogen is less electronegative than xenon. But as per the rule, we have to keep hydrogen outside.
So, xenon should be placed in the center and the remaining 4 hydrogen atoms will surround it.
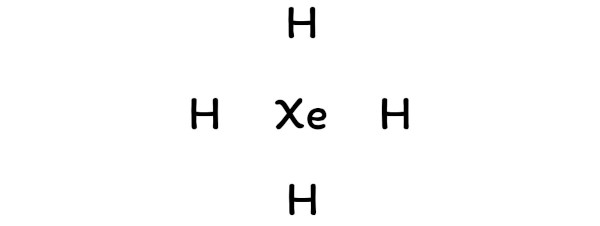
Step 2: Select the central atom
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
(Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.)
Now here the given molecule is XeH4 and it contains xenon atom (Xe) and hydrogen atoms (H).
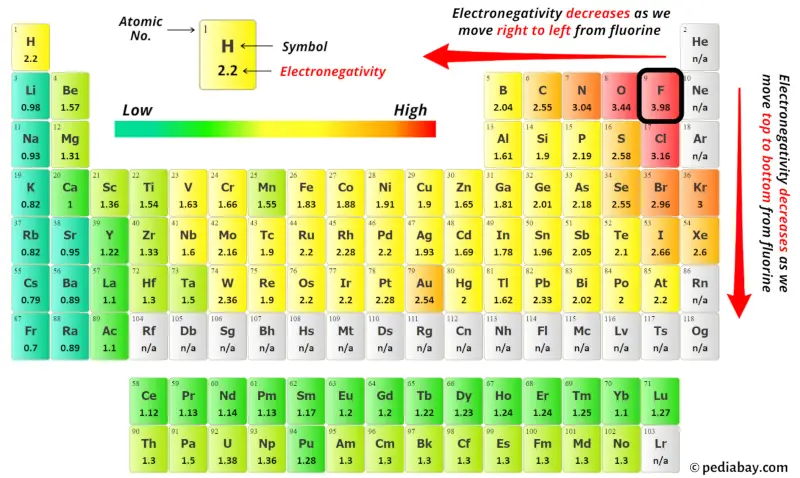
You can see the electronegativity values of xenon atom (Xe) and hydrogen atom (H) in the above periodic table.
If we compare the electronegativity values of xenon (Xe) and hydrogen (H) then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside.
So here the xenon atom (Xe) is the center atom and the hydrogen atoms (H) are the outside atoms.
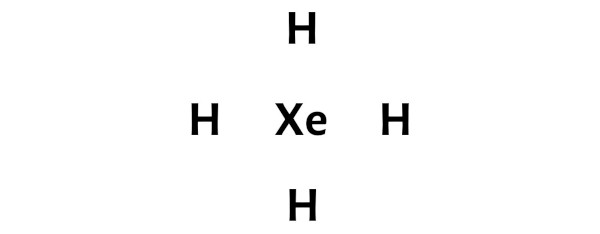
FAQ
What is the Lewis structure for XeH4?
How many valence electrons does XeH4?
How many lone pairs are in XeH4?
What is molecular geometry of xef4o?